If you want to have a good book especially for your school but you don't have a lot of money, maybe this way can be use to solve your problem. Oxford university Press give free 5 books every year for student, academics, teacher and lecture. How to get it? let's follow this step:
1. Register at OUP by sending your e-mail, and press continue
2. Complete the following fill, and press continue
3. Complete the following fill, and press continue
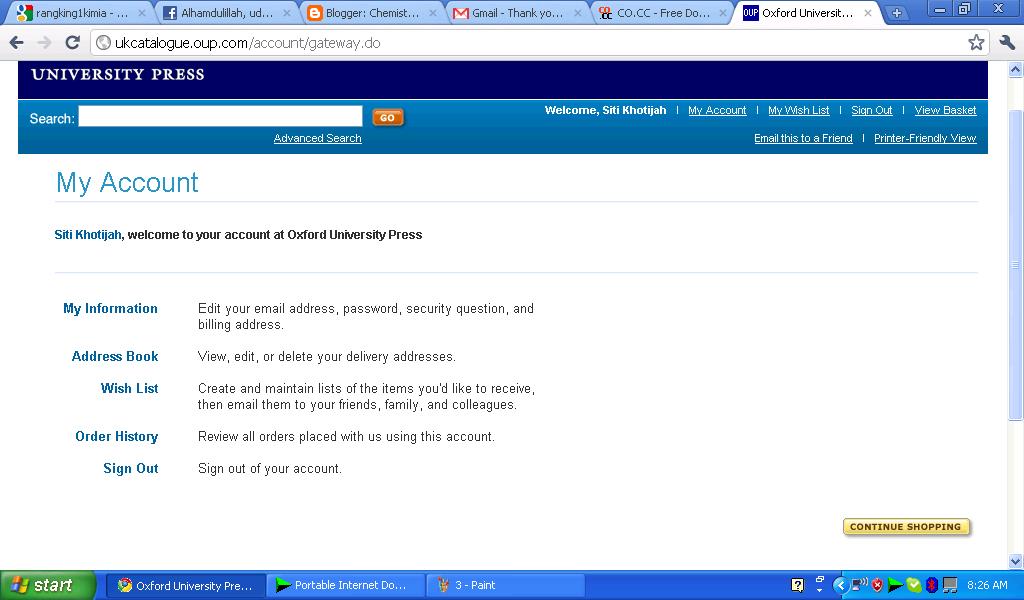
4. You will enter at Oxford University Press Home and press continue shoping
5. Click search and then write the topic what you want..for example chemistry and press go
6. Choose book what you want, NOTE: only paperback book that give for free, and press it
7. Press order an inspection copy
8. Choose evaluation for adoption as a textbook for a University or other Higher Education course and press continue
9. Finish.....
I Hope this information usefull for all
Read more »
1. Register at OUP by sending your e-mail, and press continue
2. Complete the following fill, and press continue
3. Complete the following fill, and press continue
4. You will enter at Oxford University Press Home and press continue shoping
5. Click search and then write the topic what you want..for example chemistry and press go
6. Choose book what you want, NOTE: only paperback book that give for free, and press it
7. Press order an inspection copy
8. Choose evaluation for adoption as a textbook for a University or other Higher Education course and press continue
9. Finish.....
I Hope this information usefull for all

 6:42 PM
6:42 PM
 The Teacher
The Teacher